
This aqueous extract of CGF was sterile filtered through a 0.22 μm syringe filter prior to use for all studies. Characterization of CGF Determination of Proximate Crude Composition of CGF. Direct protein quantification methods such as Bradford and Lowry assays are inaccurate for plant-based extracts (Biancarosa et al., 2017). Therefore, to

Syringeless and Syringe Filters Syringe filters and membranes for the removal of interfering materials and fine particles prior to injection; includes filters both with and without syringes; suitable for both aqueous and aqueous-organic samples; often constructed from PVDF or PTFE.
Antibodies & Protein Biology Antibody Production & Purification; Electrophoresis, Western Blotting and ELISA

Feb 12, 2021 · Prepare a 10 mM stock solution by dissolving 20 mg of Y-27632 powder in 6.24 ml of sterile pyrogen-free water. Filter the solution through a syringe filter, aliquot the solution and store aliquots

View our Sterile Syringe and Syringeless Filters products at Fisher Scientific.

Described is a process for making a dry and stable hemostatic composition, said process comprising a) providing a first component comprising a dry preparation of a coagulation inducing agent, b) providing a second component comprising a dry preparation of a biocompatible polymer suitable for use in hemostasis, c) providing said first component and said second component in a combined form in a

Apr 21, 2021 · RT&L FOCUS AREA(S): General Warfighting Requirements (GWR) TECHNOLOGY AREA(S): Air Platforms The technology within this topic is restricted under the International Traffic in Arms Regulation (ITAR), 22 CFR Parts 120-130, which controls the export and import of defense-related material and services, including export of sensitive technical data, or the Export Administration Regulation (EAR), 15

Jul 21, 2021 · The vaccine production process can be broken down into 4 stages of production – mass production of the DNA template, mass production of mRNA using the DNA template, stabilisation of the mRNA by encapsulation within a lipid nanoparticle and finally preparation of the finished product for delivery (e.g. vial for delivery via syringe).

MilliporeSigma Millex-GP Sterile Syringe Filters with PES Membrane – Ready-to-use syringe-driven units for sterilizing sterile solutions. Shop Merck Medical Millex™-GP Merck Medical Millex™-GP Syringe Filter Unit, 0.22 μm Medical Millex-GP Syringe Filter Unit, 0.22 μm, polyethersulfone, 33 mm, sterilized by gamma irradiation

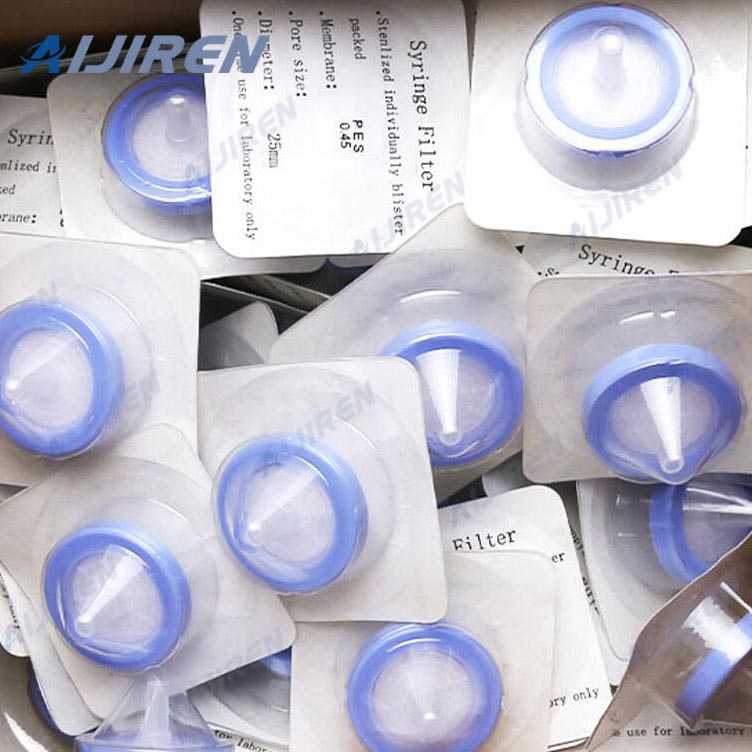
Ready-to-use syringe-driven units, designed for sterilizing and clarifying sterile solutions Shop Merck Millipore Millex™ Sterile Syringe Filters: PES Membrane Merck Millipore Millex™ Sterile Syringe Filters: PES Membrane - Clear PES; Easter housing; Pore size: 0.22μm; Dia.: 25mm; 50/Pk.

The product line includes pore sizes ranging from 0.1 μm to 5.0 μm to ensure a comprehensive selection of syringe filters are available. Syringe filters are sterilized by gamma irradiation, preventing the contamination risk that can result from ethylene oxide sterilization. They are individually blister packed to ensure sterility during storage and handling. Replacing a syringe filter midway through the process may lead to contamination, spills, and workflow disruption.

Available in 4, 13, 25, 33, and 50 mm diameters with a variety of membranes, Millex ® sterile syringe filters are ideal for sterilizing organic solvents, aqueous solutions or air/gas. Many sample preparation methods specify Millex® filters because of their unsurpassed quality and consistency. Millex ® filters not only feature minimal hold-up

Ready-to-use, syringe-driven units designed for sterilizing and clarifying sterile solutions are available in 0.1, 0.22, 0.45 and 5.0μm pore sizes

Use Technical Service Consultants™ Hygiene Sponge Sampling Kit enables sampling in difficult to reach places, such as crevices and corners. It comes pre-dosed with a neutralizing buffer, swab heads, shaft materials,,tubes for convenient storage and handling and medical-grade plastics for assured quality. .